
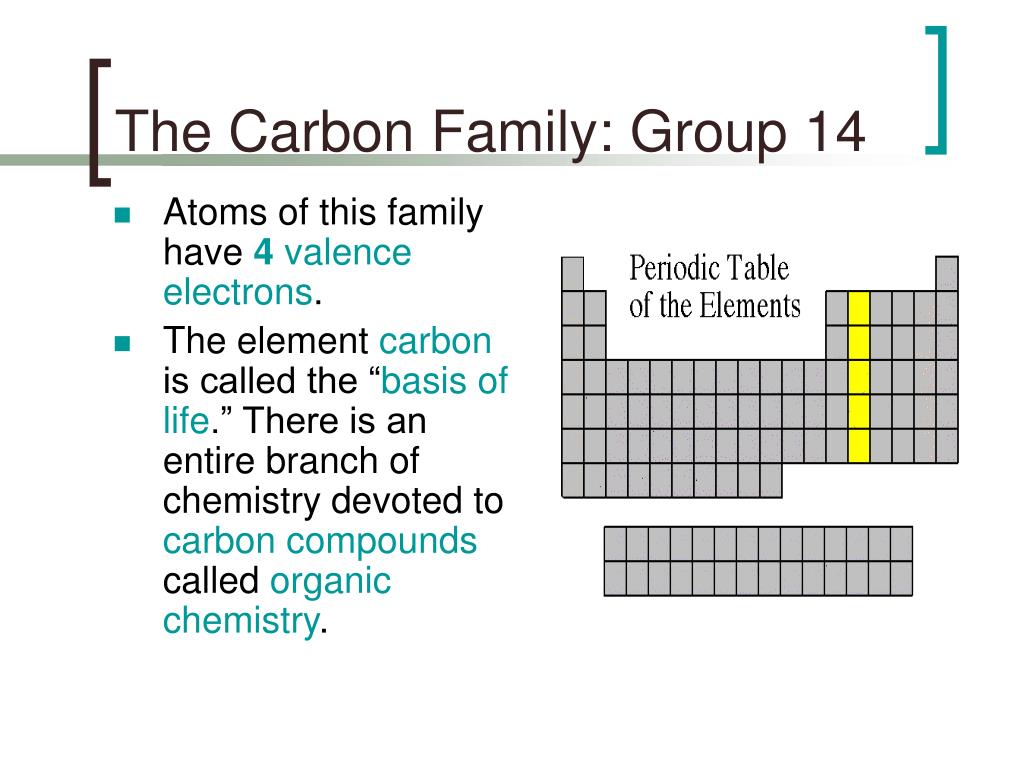
Because Li has an electron in the n = 2 shell, it is larger than H or He whose 1 s electron clouds are much closer to the nucleus. The area covered by electrons in boron is larger than carbon but still smaller than beryllium.īy far the largest atom illustrated in these color plates is Li. It also has the circular area in the center which is highly concentrated with dots. The electron density diagram for Carbon has four lobed shape region pointing to each side of the axis that are highly concentrated with dots. This lobed region is also highly concentrated with dots.

The electron density for boron shows a circular area concentrated with dots but there are also two lobed area symmetrical about the vertical axis. The circular area for beryllium is the largest since the electrons are more spread apart. The electron density diagram of Hydrogen and helium are superimposed on the diagram for lithium to better highlight the difference in sizes. Lithium has a 2 s orbital which translates to a significantly larger circular area in which the electrons are dispersed. Hydrogen has a larger circular area concentrated with dots when compared to helium. On the right from top to bottom are Beryllium, Boron, and Carbon. On the left from top to bottom are Hydrogen, Helium, and Lithium. \) The figures above show the electron density of different elements.


 0 kommentar(er)
0 kommentar(er)
